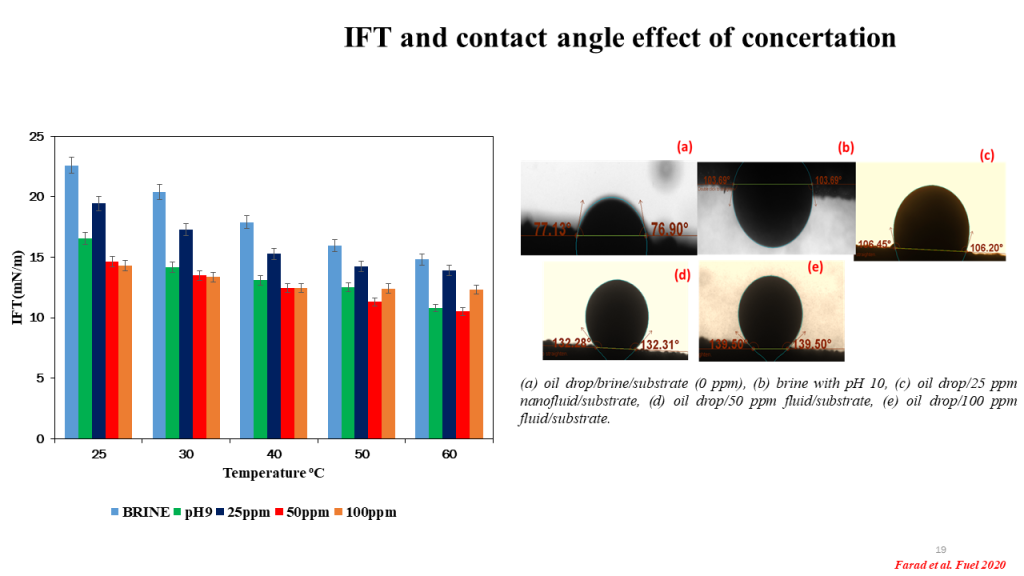
Interfacial Tension (IFT): Interfacial tension refers to the force that exists at the interface between two immiscible fluids. It is a measure of the energy required to increase the surface area of the interface. The IFT is influenced by the nature of the two fluids and the temperature. It is commonly denoted by the symbol γ (gamma) and has units of force per unit length (e.g., dyn/cm or N/m).
The IFT plays a crucial role in various phenomena, such as the behavior of droplets or bubbles, emulsion stability, and the displacement of one fluid by another in porous media. Lowering the interfacial tension can enhance mixing or promote the spreading of one fluid on another.
Contact Angle: The contact angle is a measure of the wettability of a liquid on a solid surface. When a droplet of liquid comes into contact with a solid surface, it can either spread out or form a distinct shape. The angle formed between the liquid-solid interface and the tangent to the droplet at the three-phase contact line is known as the contact angle.
The contact angle provides information about the degree of interaction or adhesion between the liquid and the solid surface. A high contact angle (typically greater than 90 degrees) indicates that the liquid does not wet the surface well, resulting in a more rounded or spherical droplet. Conversely, a low contact angle (typically less than 90 degrees) suggests good wetting, and the droplet tends to spread out and form a flatter shape.
The contact angle is influenced by various factors, including the surface tension of the liquid, the surface energy of the solid, and the presence of impurities or coatings on the solid surface.
Effect on Concentration: The interfacial tension and contact angle can both have an effect on the behavior of fluids in terms of their concentration and distribution. For example, in the case of two immiscible fluids, a lower interfacial tension can promote the mixing and dispersion of one fluid into the other, leading to a more uniform distribution of components.
Similarly, the contact angle can influence the spreading or absorption of a liquid on a solid surface. A lower contact angle allows for better wetting and penetration of the liquid into the solid, potentially leading to increased concentration or absorption of the liquid into the solid material.
Overall, both interfacial tension and contact angle are important factors in understanding and predicting the behavior of fluids in relation to concentration and distribution in various systems.